The FDA has been busy this month. Last week they advised on The Dangers of Skin Peels Without Professional Supervision. This week, the Food and Drug Administration has approved ARS Pharmaceuticals’ Neffy epinephrine nasal spray.
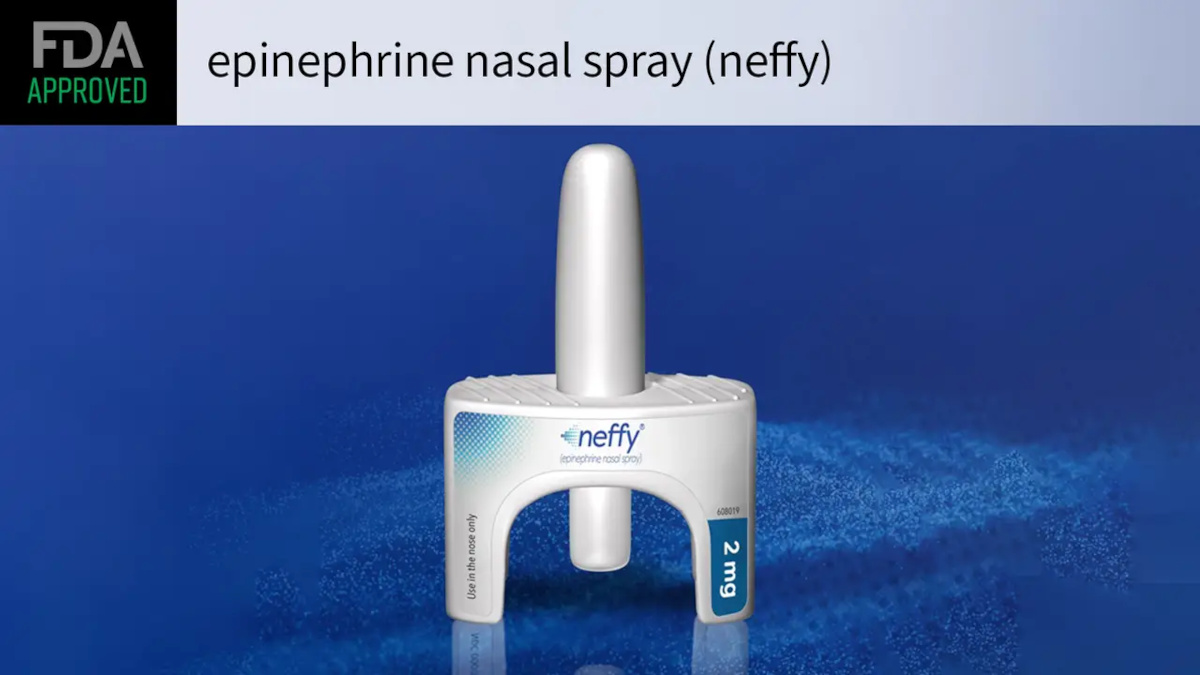
Neffy nasal epinephrine spray is now FDA approved for anaphylaxis (severe allergic reactions).
Neffy is for the Treatment of Severe Allergic Reactions – Anaphylaxis
Anaphylaxis is a life-threatening, severe allergic reaction that in severe cases causes swelling of the airway and suffocation. This can be caused by bee stings, latex as well as food and drug allergies. Neffy is the first epinephrine product for the treatment of anaphylaxis that is not administered by injection.
No Needles is Big News and a Game Changer
No needles is big news and ideal when treatment would otherwise be delayed by Trypanophobia — an intense fear of needles. The FDA approved the Neffy epinephrine spray for adults and children over 66 pounds. It is administered as one 2-milligram spray into a nostril. Each Neffy package has two epinephrine nasal sprayers. If a reaction does not resolve quickly, the second spray is administered into the same nostril. The amazing part is levels of epinephrine in the body after nasal administration match the levels seen with injection.
The EpiPen – the Gold Standard for Years
Currently, epinephrine is delivered via injection. The most popular brand name device goes by the name EpiPen. It is a spring loaded device that injects epinephrine with the push of a button.
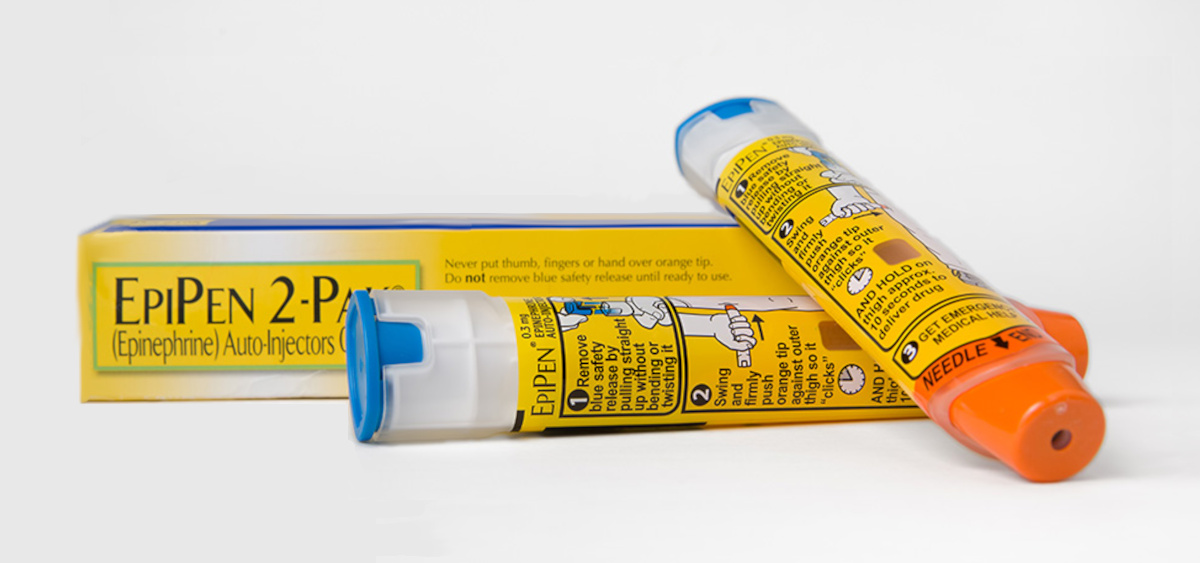
The EpiPen has a guarded needle (in the orange cap) at one end and a blue push-button at the other end to control injection.
While safe and effective, there have been some injuries reported, primarily from the sharp needle. In addition, storage at the temperatures typically seen in a California car in the summer, can make the pen inoperable. Over the last decade, there have been numerous shortages of EpiPens from the manufacturer Pfizer; however, the biggest obstacle to early use is the fear of needles, especially common in children.
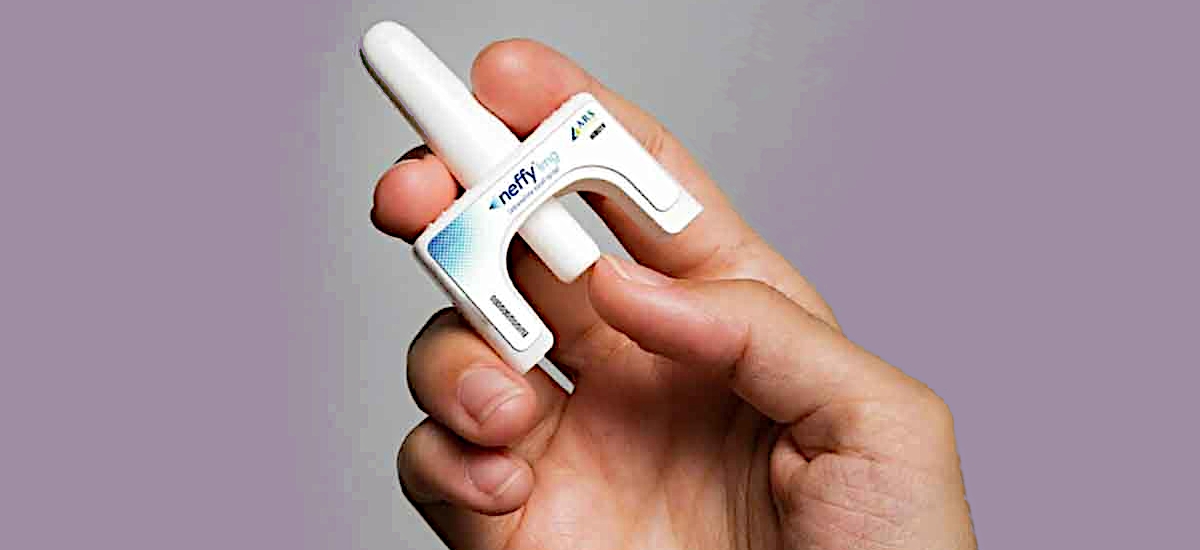
The Neffy nasal spray injector is simple to use. Simply place the blunt end in the nostril and depress the button with your thumb.
The Neffy nasal spray does not require a needle to deliver the epinephrine. The blunt end is placed into the nostril and the thumb is used to depress the button. Do not sniff during or after receiving a dose of Neffy. The prescribing information is available here:
How Long Can You Store Neffy?
Shelf life is another issue. Epipen Autoinjectors usually have a shelf life of 12 to 18 months, but always check the label for the expiry date. Neffy’s shelf life is 30 months.
Neffy is also stable at higher temperatures than epinephrine in auto-injectors. ARS Pharma’s research finds the medication can be stored at up to 122 degrees Fahrenheit for up to three months and still maintain much of its potency. However, it’s still recommended that Neffy be stored at room temperature, between 68 and 77 degrees F. The label says, “storage at high temperatures of up to 122 degrees Fahrenheit is allowed for a few days.” This is important if the product is left in a parked car. For those who live in colder climates, the label advises against freezing. However, if accidentally left in the car overnight, the injector can be used after it returns to room temperature.
Full prescribing details for Neffy are available on the ARS Pharma Website.
Neffy’s Approval in Younger Kids
EpiPen also has EpiPen Jr. for smaller humans. ARS Pharma plans to submit a supplemental new drug application to the FDA in the coming weeks for approval of a 1 mg sprayer for children weighing 30 to 66 pounds (13.6 to 30 kgs). The FDA will then have six months to make its decision.
Cosmetic Plastic Surgery
While today’s post has little to do with my specialty, Cosmetic Plastic Surgery, it is important information for my patients with severe allergies. As with any new medication, not all the good and bad is known yet. It is always best to consult with your doctor, and when appropriate an allergy specialist.
I am an experienced, Board Certified Plastic Surgeon. If you are considering cosmetic plastic surgery in the San Francisco Bay Area, call my office (925) 943-6353. My staff is available to answer your questions and set up a private consultation appointment in our Walnut Creek, CA, plastic surgery clinic.
Previous Post Next Post