The COVID-19 vaccines are important tools to end the COVID-19 pandemic. Safe, effective, no-cost COVID-19 vaccines will be available to everyone in California. This week they are available to everyone age 65 or older and all healthcare workers.
How To Beat COVID-19
The vaccine paired with other daily preventative health measures, such as wearing face coverings and social distancing, will slow the spread of COVID-19 so businesses and schools can fully reopen and we can return to a more normal way of life.
Contra Costa County is now making the COVID-19 vaccine available to everyone 65 and older and all healthcare workers.
Contra Costa County has administered almost 50,000 doses of the vaccine to date. They are now offering COVID-19 vaccinations for people age 65 and over and all health care workers. Here is the direct link to schedule an appointment: Contra Costa County Vaccine Appointments
COVID-19 Vaccine Distribution Phases
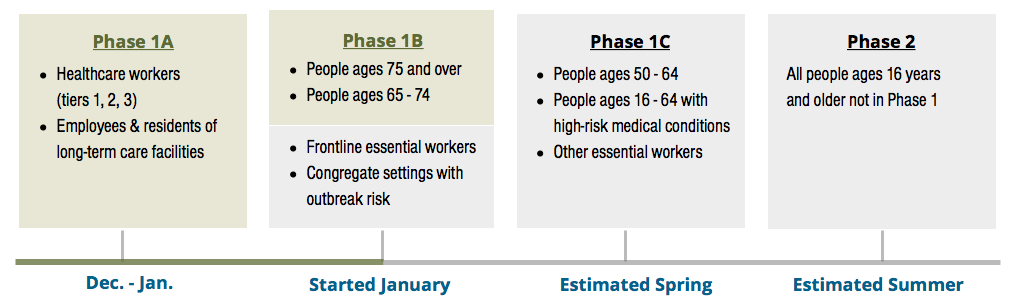
Above is a chart outlining Contra Costa County’s COVID-19 Distribution Phases and estimated rollout. This was taken from the CoCo County Health Services website.
We are currently in vaccination phase 1B. Phase 1A began in December with the availability of the first COVID-19 vaccine. Phase 1B began this month. Phase 1C is estimated to begin in the Spring, while Phase 2 should start this summer. More about CoCo County’s vaccination program is available on their Vaccine Page.
Facial Fillers and COVID-19 Vaccinations
Facial fillers are not a contraindication to vaccination You may have seen something in the news about swelling after vaccination, but to date, all swelling has been localized and resolved completely. Here are the details:
Three patients out of 15,184 receiving the MODERNA SARS-CoV-2 mRNA vaccine developed facial swelling after vaccination. The three patients were in Moderna’s Phase Three trial and developed (2) facial and (1) lip swelling. Two of the patients had a history of dermal filler placement 6 months (46-F) and 2 weeks (51-F) prior to vaccination. There was one incident of lip angioedema in a patient (29-F) with a history of lip dermal filler (unknown how long prior to vaccine). All events occurred within two days of receiving the vaccine dose. All patients reported complete resolution of these effects. There were no reported reactions in the placebo group.
The following recommendations are to serve as guidance from ASPS when discussing the administration of dermal fillers in patients who are anticipating receiving the Moderna COVID-19 vaccine and have a history of having received dermal filler injections.
- The current reported side effects have only been observed in the Moderna vaccine trial in 3 out of 15,184 recipients; it is currently unknown how many participants had a history of dermal filler usage.
- Patients should NOT be discouraged from receiving the COVID-19 vaccine; all reports of facial swelling were noted to be self-limited and resolved with oral antihistamines or corticosteroids.
- Patients should be educated with the current data available, prior to receiving dermal fillers.
- A complete medical history should be obtained prior to any medical intervention.
- Dermal filler-associated delayed hypersensitivity immunologically based reactions have been reported for both hyaluronic acid and non-hyaluronic acid-based fillers; these reactions have specifically been demonstrated after influenza-like illness.
The American Society of Plastic Surgeons will continue to monitor the scientific literature and update membership as more data is made available.
Previous Post Next Post