What we know about Breast Implant Associated Anaplastic Large Cell Lymphoma (BIA-ALCL) is evolving. We learn more as each new case is identified. Right now, there are just too few cases to know many of the specifics; however, today I will go over the data currently available.

ASPS and ASAPS summary of BIA-ALCL in 2019
Much of this information comes from the American Society for Aesthetic Plastic Surgery (ASAPS) and the American Society of Plastic Surgeons (ASPS). Thanks to its members voluntarily reporting and collecting information on BIA-ALCL, these US based national plastic surgery organizations have the best database on BIA-ALCL in the world.
What is BIA-ALCL?
Breast implant-associated anaplastic large cell lymphoma (BIA-ALCL) is an uncommon and treatable type of T-cell lymphoma that can develop around breast implants. BIA-ALCL is not a cancer of the breast tissue itself. It is not breast cancer, but it is found in the breast, around the capsule which surrounds breast implants.
When Does It Present?
The time between breast implant insertion and diagnosis of BIA-ALCL varies greatly. It ranges from 9 months to 27 years, with an average delay in presentation of 9.2 years.
Who Gets BIA-ALCL?
Cases seem to be concentrated in patients who have, or who have had textured breast implants. It seems to be related to the aggressiveness of the texturing and has occurred in patients with both silicone and saline filled breast implants.
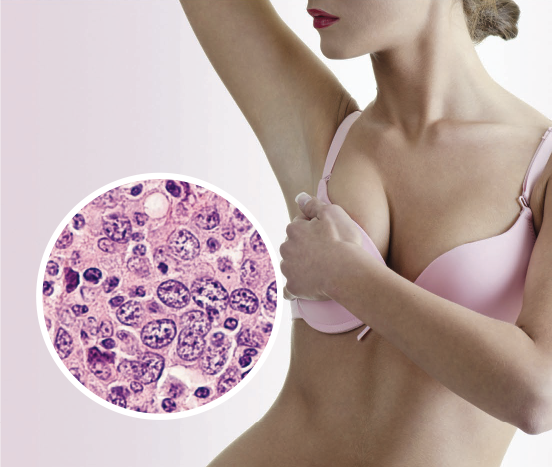
When in doubt; check it out. Early diagnosis and treatment are key to curing BIA-ALCL.
After reviewing all available case series, case reports, and registries, BIA-ALCL is more common with textured implants. Textured implants are used less frequently than smooth implants. Textured implants are also used more often for breast reconstruction after breast cancer, because shaped implants are more desirable in this population and texturing is used to reduce breast implant rotation for shaped implants.
To date, no cases of BIA-ALCL have been verified in patients who have had exclusively smooth breast implants. However, it is not possible to exclude the appearance of BIA-ALCL in association with smooth implants at this time. The FDA reports that they are aware of smooth breast implant only cases; however, they warn that this information is “unverified” and potentially “inaccurate.”
The association of BIA‐ALCL and textured implants may be related to the increased surface area of the texturing; however, this has not yet been definitively proven. The variation in surface texturing among breast implant manufacturers may mean there are variable risks for the development of BIA-ALCL.
How Does BIA-ALCL Present?
The majority of patients present years after their initial surgery with one breast gradually increasing in size. The increased size is from fluid, serum, collecting around the breast implant. This collection of fluid is called a seroma. Seromas are normal right after surgery; however these seromas appears later and are thus called a delayed seromas. A few patients have presented with different symptoms such as a mass, skin rash, fever and night sweats, and lymphadenopathy.
How Is The Diagnosis of BIA-ALCL Made?
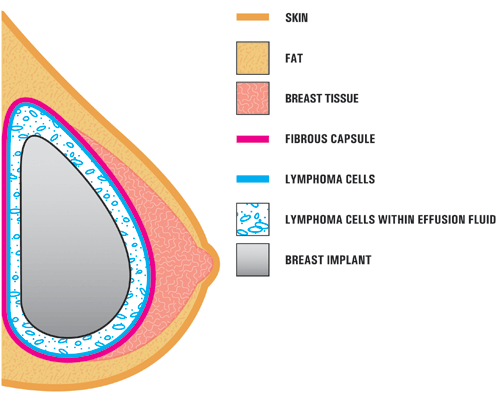
BIA-ALCL usually presents as increased breast size due to fluid collecting around a textured breast implant.
Diagnosis is based on analysis of the fluid in the seroma. Most commonly, ultrasound‐guided fine needle aspiration of the peri-implant fluid is assessed with immunohistochemistry for CD30-positive large anaplastic T-cell lymphocytes.
How Is BIA-ALCL Worked Up?
PET‐CT is performed following a positive diagnosis. Mammograms are not helpful for evaluating lymphoma, but are important for the evaluation of breast cancer. Often, a multidisciplinary team approach including, when required, an oncological breast surgeon and an oncologist specializing in lymphoma.
How Is BIA-ALCL Treated?
The treatment of BIA-ALCL is evolving. In most cases, cure is obtained by removal of the breast implant and the capsule surrounding it. Incomplete capsular resection has been associated with both recurrence and significantly lower survival. Rarely, patients may present with a mass and have an increased risk of requiring radiotherapy and chemotherapy. Treatment approach should follow international guidelines established by the National Comprehensive Cancer Network (NCCN) for BIA-ALCL, available at nccn.org.
Current treatment recommendation is for bilateral complete capsulectomy and implant removal, as a small number of women have had contralateral disease found incidentally; however, it cannot be stressed enough that the treatment is still evolving, and each patient must be individually evaluated. If you suspect you have BIA-ALCL, do not delay, and contact your plastic surgeon or primary medical doctor immediately.
Summary Statement On BIA-ALCL From The ASPS
I have included below a statement released by the American Society of Plastic Surgeons (ASPS) this week. It summarizes well what we currently know about BIA-ALCL. The ASPS has also published an 2019 online BIA-ALCL summary.
“Breast implant-associated anaplastic large cell lymphoma (BIA-ALCL) is an uncommon type of lymphoma that can develop in the scar capsule near saline or silicone breast implants. This disease is currently being investigated as to its relationship with breast implants. The family of ALCL is a rare cancer of the immune system, which can occur anywhere in the body. Based on adverse event reports, the United States Food and Drug Administration (FDA) estimates the total number of cases of BIA-ALCL to be over 450 cases.”
“It has been noted that the majority of BIA-ALCL patients have a history of a textured-surface device. An exact single-number estimate of the risk for both textured and non-textured implants is not possible with the currently available data. Lifetime risk of BIA-ALCL has been estimated at 1:1,000 to 1: 30,000 for women with textured breast implants, and BIA-ALCL risk is currently under investigation. BIA-ALCL usually involves swelling of the breast at an average of 3 to 14 years after the initial breast implant operation. Most cases were cured by removal of the implant and the capsule surrounding the implant; however, rare cases have required chemotherapy and/or radiation therapy for treatment.”
“Patients with breast implants should be followed by a surgeon over time and seek professional care for implant-related symptoms such as pain, lumps, swelling, or asymmetry. Patients should monitor their breast implants with routine breast self-exams and follow standard medical recommendations for imaging (e.g. Mammography, Ultrasound, MRI). Abnormal screening results or implant-related symptoms may result in additional expenses for tests and/or procedures to properly diagnose and treat your condition. Tests and procedures could include but may not be limited to: obtaining breast fluid or tissue for pathology and laboratory evaluation, surgery to remove the scar capsule around the breast implant, implant removal, or implant replacement.”
Previous Post Next Post